NEW
Product Assembly Services
Before a medical device or pharmaceutical product can enter pre-clinical evaluation, it must first exist as a consistent and reliable physical product. At this decisive moment in development, product assembly transforms design intent into tangible reality shaping how a product will be tested, refined, validated, and ultimately industrialized.
Cleaning & Reprocessing
In regulated manufacturing environments, cleaning and reprocessing play a critical role in ensuring that materials and products can safely and consistently progress through downstream operations. These activities directly support product quality, process reliability, and regulatory compliance across a wide range of healthcare manufacturing services.
Medistri’s Integrated Manufacturing: From Assembly to Final Delivery
For a product to reach its final users in full compliance with its specifications, assembly and finishing activities must take place in a controlled and well-defined environment. From order intake through final delivery, Medistri follows a structured and validated manufacturing workflow designed to ensure consistency, quality, and regulatory compliance at every stage.
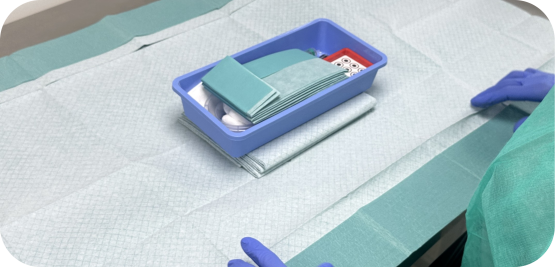
Customized Assembly for Startups
At Medistri, we specialize in the precision-driven assembly, packaging, and sterilization of medical devices or pharmaceutials, catering to both emerging startups and established companies. Our process is designed to support the unique needs of each client, from prototype development to routine large-scale production.
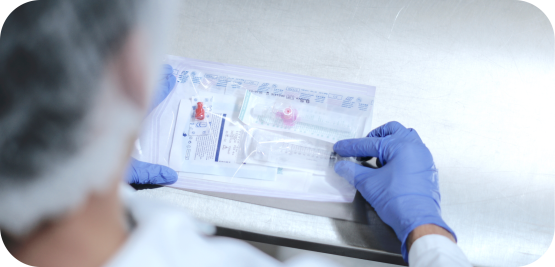
Medistri's Customized Single-Use Surgical Instruments
Created for start-up companies and early-stage products, Medistri's manufacturing team can offer primary, secondary, and tertiary packaging solutions that fit your strategic & regulatory requirements.
Medistri’s Manufacturing Services
Created for start-up companies and early-stage products, Medistri's manufacturing team can offer primary, secondary, and tertiary packaging solutions that fit your strategic & regulatory requirements.
Medistri’s SwissMedic GMP Compliance
Good Manufacturing Practices (GMP) are vital standards in the pharmaceutical industry that ensure product quality and safety throughout production. These regulations protect against contamination, errors, and deterioration, ensuring that healthcare products consistently meet strict standards.
Our Assembly Process. In 6 Steps.
Medistri follows a precise protocol in order to deliver the optimal final outcome within weeks of our collaboration. With Medistri’s GMP authorisation, you can now also introduce pharmaceutical products into your personalised kits.